
Drew Weissman, M.D., Ph.D. in his lab at Perelman School of Medicine at the University of Pennsylvania.
By Bridget Velasquez
Like most young kids in Lexington, Drew Weissman grew up in Lexington playing with friends in the neighborhood, avoided trouble, and moved through the Lexington school system graduating with the Class of 1977. While his years in Lexington were seemingly unremarkable, everything he has done since has been nothing short of miraculous. Searching for a cure for a nephew’s affliction, Drew’s research has become the cornerstone in the development of vaccines that will save millions of lives.

Lexington High School Class of 1977.
Weissman’s research as a professor of medicine at UPenn is the foundation for the mRNA COVID-19 vaccines developed by both Moderna and Pfizer. But, Dr. Weissman’s original motivation was not to cure a future pandemic, it was to cure his nephew.
His nephew, Max, has something called X-linked agammaglobulinemia (XLA). This is a genetic disorder caused by a mistake in the Bruton’s Tyrosine Kinase (BTK) gene. Although it’s a mistake in a single gene, it has catastrophic consequences – Max cannot properly develop his own B cells, which means that his immune system cannot properly develop antibodies. To protect himself from everyday viruses and bacteria, he has to get immunoglobulin infusions made from serum from donated blood. These infusions give Max antibodies, and he has been dependent on these infusions for almost his entire life. XLA is on the X chromosome, so if Max has sons, he won’t pass it down. But he will pass it down to his daughters. His daughters will become carriers – meaning that they won’t express the genetic defect and need immunoglobulin infusions, but they will have the defective gene and could pass it down to their children. If their sons get the gene, they will express it and will need immunoglobulin infusions like Max.
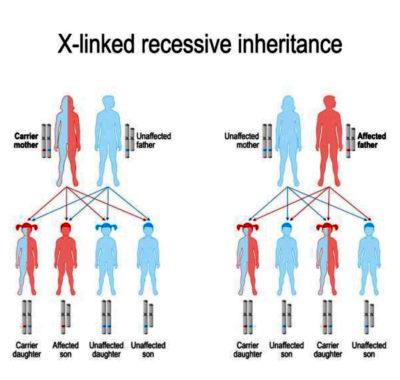
This genetic disorder could last for generations, and there is no cure.
No cure yet, anyway, but Drew Weissman plans to change that. When Max graduated high school, Drew approached Stephanie Weissman (his sister and Max’s mother).
“Drew would never tell me anything unless he knew it was going to happen or was very sure,” Stephanie said, “and he told me, ‘I think I’ll be able to cure Max.'”
Stephanie believes in her brother.
“I don’t know how long it will take, but I believe he will come up with a cure for my son,” she said.
__________
According to Stephanie, Drew was a very responsible child who “never got into trouble in his life” (he actually got into some mischief at least twice). When he was only six years old, their mother trusted him to bike to the library without being distracted and going elsewhere – even at six years old, Weissman enjoyed the library.
They had typical childhood experiences. Their mother would get on them to clean their rooms, and they would shove things under the bed to make things look clean. They would argue, but only when their mother was watching. They would accidentally get locked out of their house and have to climb in through the window to get inside after school. But even as a child, Weissman showed great promise.
“I don’t think I ever questioned what he was going to do,” Stephanie said, “I knew he was going to do something big that would change the world.”
Weissman was always interested in all sciences, but he also had great potential as an engineer. His father was an engineer, and he often did personal projects with his children. Weissman was a natural, but unfortunately, one of these projects would put him at odds with the local constabulary.
When Weissman was around 12 years old, he, his dad, and his sister built a hydroplane boat. It was small (built only for a single person) and was powered by a motor. They took it to a lake in New Hampshire, and the police caught Drew the very first time he used it.
“It was a minor thing,” Stephanie said, “We did not have a New Hampshire boating license. In Massachusetts, you did not need a license for a motor less than 10 hp.”
A minor thing, indeed. It had no long-term impact on Weissman. And today, it’s a story that Stephanie enjoys telling journalists when they ask for fun stories about her brother. It was one of two times she said he got into trouble – the other time was when she knocked over the Christmas tree and blamed her brother.
Despite his promise as an engineer, Weissman’s mother tried to dissuade him from studying engineering. By the time he graduated from Lexington High School in 1977, engineers were being laid off in big numbers throughout the country. So, he changed course and chose pre-med. He earned both his Bachelors in Biochemistry and Masters in Enzymology from Brandeis University in 1981. Master’s degree in hand, he attended Boston University and earned a medical degree (M.D.) and Ph.D in Immunology in 1987. Afterward, he got a fellowship researching HIV at the NIH under Anthony Fauci (for reference, 1987 is when WHO launched The Special Programme on AIDS, President Ronald Reagan gave his first speech regarding the HIV/AIDS crisis in the U.S., and the first AIDS medication gained FDA approval. More than 50,000 AIDS cases had been reported in the U.S.).
Not long after graduation, Dr. Weissman accepted a position at the University of Pennsylvania and has been there ever since. It was here that he met Katalin Kariko, and the two would begin their mRNA research in earnest. The two of them met entirely by happenstance – there was no formal introduction, no third party who thought their research would complement each other.
The two met by arguing over the photocopier.
“This was back in the old days when the only way you could read an article in a journal was to photocopy it,” he said, “So [Kariko] and I used to fight over the machine.” For the next few months, the two chatted while waiting for their turn with the photocopier. The two learned about the other’s current research – Kariko was working with mRNA and Weissman was working with a cell of particular importance in vaccines. Realizing the complementary nature of their research, the two teamed up for experiments and research.
Their research was riddled with challenges. It wasn’t necessarily that mRNA is unusually difficult to work with – it’s that it killed the mice upon injection. The mRNA caused intense swelling and a catastrophic immune system response. The issue was that the regular mRNA looked too much like a viral genome. When injected, the regular mRNA elicited pathways that shut down protein translation – and if the immune system response was aggressive enough, it would kill the cell.
The research was slow.
A few years later in the early 2000s, Weissman’s and Kariko’s work paid off. They discovered how to keep the mRNA under the immune system’s radar by slightly editing one of mRNA’s four nucleic acids. It no longer looked too much like a viral genome and, as a result, the immune system no longer attacked it. Weissman and Kariko published their findings in 2005.
 mRNA and Regenerative Biology
mRNA and Regenerative Biology
Weissman’s and Kariko’s findings have had far-reaching impacts. In 2008, a team of stem cell biologists, including Dr. Derrick Rossi, were trying to use mRNA to induce pluripotent stem cells from adult stem cells. Pluripotent stem cells are incredibly valuable and promising in regenerative biology research, because they can give rise to any body cell, including neurons and heart tissue. However, at that time, these cells were found only in human embryos, so research was highly controversial. If the team could induce pluripotent stem cells from adult cells, scientists would no longer need to rely solely on embryonic tissue. The research was frustrating because when the team injected the mRNA into adult cells, the adult cells died. The team was at a dead-end – that is until they found Weissman’s and Kariko’s 2005 paper.
“Finding Kariko and Weissman’s paper was really the key moment,” Dr. Rossi said, “What I recognized is that we could use this technology to synthesize any protein and have the cell synthesize any protein.” Dr. Rossi co-founded Moderna in 2010 to use this technology to develop therapeutics. Today, Moderna has 20 mRNA vaccines in development and is one of the leading manufacturers of the COVID-19 vaccines.
mRNA and CRISPR-Cas9
mRNA is not limited to vaccines – it also shows promise for gene therapy. CRISPR-Cas9―a genome editing technology― also shows promise, however mRNA and Cas-9 have some fundamental differences. According to Dr. Weissman, CRISPR-Cas9 is primarily used to remove parts of a gene, while mRNA is designed to add to a gene. CRISPR-Cas9 also edits and alters the genome itself, but mRNA does not.
“With CRISPR-Cas9, the good thing is that it alters the genome, but the bad thing is that it alters the genome,” Dr. Rossi explained somewhat tongue-in-cheek, “And I would argue that mRNA is almost surely going to be a safer therapeutic paradigm because it doesn’t alter the genome.”
When directly editing genetics with CRISPR-Cas9, scientists must be careful of unforeseen consequences and off-target effects, such as unintended genetic deletions or unintended genetic insertions. Genetic deletions or insertions cause frame shift mutations, and these mutations can contribute to the development of a variety of genetic disease, including Tay-Sach’s disease and Smith–Magenis syndrome (there is a beneficial deletion though – a particular deletion decreases the risk of getting a certain strain of HIV).
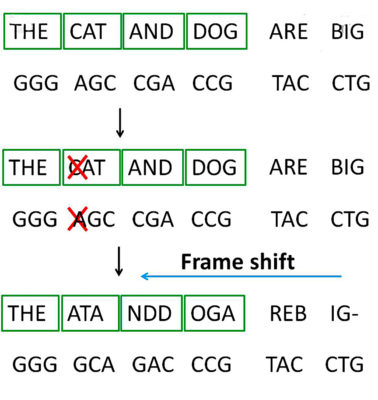
Genetic deletions or insertions cause frame shift mutations, and these mutations can contribute to the development of a variety of genetic diseases.
Dr. Rossi explains that mRNA doesn’t have this concern, because it’s transient and degradable – the mRNA creates the protein that you want it to create, then the mRNA degrades, and then the protein would degrade as well. Although this requires multiple injections, it is much safer.
mRNA and the COVID-19 Vaccines
The COVID-19 pandemic and mRNA vaccines threw Weissman and Kariko’s work into the center of public attention.
The mRNA vaccines differ from traditional vaccines. In a traditional vaccine, you get injected with a weakened or inactive strain of that particular virus. Your immune system sees the invader and creates a new, specialized antibody to destroy it. Your body learns how to destroy the virus before you encounter it in real life, so that if you do encounter it, your body knows what to do. Unfortunately, it can be difficult and expensive to manufacture weakened or inactive viruses to use in vaccines.
In a modified mRNA vaccine, the modified mRNA is designed to slip into cells and instruct the cell to make specific, desired proteins. For the COVID-19 vaccine, the mRNA will instruct your cells to create a “spike protein” – a harmless protein found on the surface of the COVID-19 virus. Because mRNA is transient, it gets broken down when your cells make the spike protein. Your immune system recognizes that your cells aren’t supposed to have the spike protein and creates antibodies to destroy the cells. You will now have COVID-19 antibodies.
Weissman and Kariko’s work is the fundamental foundation for the mRNA vaccines. Without their work, we would not have the vaccines.
“I believe that Weissman and Kariko deserve the Nobel Prize in Chemistry,” Dr. Rossi said, “Without their key discovery, we wouldn’t have the COVID vaccines right now.”
If they hadn’t made their discovery then, maybe it would’ve been found eventually by another researcher. But that hypothetical researcher would’ve made the discovery later, and so subsequent research would have happened later, and so the technology required to make a COVID-19 mRNA vaccine would have happened later as well. According to Dr. Rossi, overcoming the pandemic would be on an entirely different timescale.
__________
Weissman has been incredibly busy since the pandemic started. While he is not directly involved with making American COVID-19 vaccines, he is working with Thailand to develop theirs (Weissman has a history of collaborating with Thai scientists going back several years).
According to Weissman, Thai scientists are concerned about vaccine accessibility – they’re concerned that it could take years for them and their neighbors to see the arrival of a Western vaccine.
“The U.S. is trying to grab as many [vaccine] doses that they can, and Europe is doing the same thing. The pharmaceutical companies sell the vaccine to the people who are able to pay the most,” Weissman says, “That leaves Thailand, Burma, Vietnam, Pakistan – all of those countries with very limited access and difficulty getting the vaccines.” Population is also an issue. China has a population of about 1.4 billion, and the U.S. has a population of 328 million – a vaccine developed by a country is likely to go to its own citizens first, and then exported.
Weissman is committed to helping Thailand.
“He shared his technology with us because he wants Thailand and low-income nations to have access to the crucial vaccine,” said Dr. Kiat Ruxrungtham, chair of Chulalongkorn University’s Chula Vaccine Research Centre, explained to Thai PBS World in June, “Dr. Weissman has been a true partner, also attending online meetings with us every week.”
__________
The pandemic has kept Weissman both very busy and very sleep deprived. He used to enjoy martial arts, going to the theatre, and kayaking, but has not had time since the pandemic began.
Still, Weissman enjoys working in science and doing research and the creativity is his favorite part.
“It’s the creativity – with research, you have to evaluate all of the data and then the trick is to figure out what is going on and then develop your own hypotheses to test and see if they’re correct, and see if they make a new, interesting finding.”
__________
Despite being a doctor for humans, Drew Weissman’s office at the University of Pennsylvania is located at the Hill Pavilion Veterinary Medicine Teaching Research Building. Several other medical research buildings are nearby, probably because of the close association with animals in medical research. There are no framed accolades in his office – just a photo of his family and a sculpture his oldest daughter made for him.
Weissman’s goal for 2021 is to continue his mRNA research and advance the research he and his team are working on to find a simple-to-deliver cure for sickle cell anemia.
While his research has spawned vaccines that will no doubt save millions of lives, he keeps his focus simple and true. “If we can develop this, curing Max will be much easier,” he said.
To that, we say amen.

